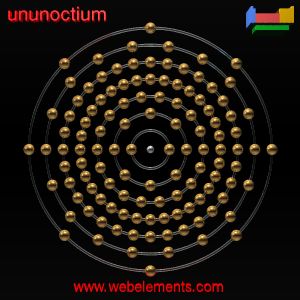
Ununoctium
Janet Kuypers
from the “Periodic Table of Poetry” series (#118, Uuo)
11/30/12
I first only heard of you a decade ago.
You seemed so reactive, so unstable,
and yet I was so attracted to you.
I should have known better.
I should have known that
your radioactive personality would
cause your destruction, so I guess
I’m glad I’m not around to see it.
I have only seen you three or four times
since you started to self-destruct,
so from afar I can only guess
what you’re made of, or what you can do.
But still, I can’t get you out of my mind,
so I’m left here to guess about you,
based on what little I could ever infer
about you. This is all you leave me.
When I saw you before, you seemed
kind, and noble when you were with me...
But that was before I saw what you
were made of, how hard you could be.
So much emanated from you with me,
but you’ve systematically shattered
any preconceived notions of who you are,
that I don’t even know what to believe.
You’re that explosive, and I’ve been
unsuccessful in any attempts to synthesize
with you... It’s funny, you seem
like you want to be discovered,
but I can only predict, calculate, or
extrapolate what I think you can do.
If only you would let me crack your shell
so I could see what you’re made of...
• also known as eka-radon.
• radioactive, very unstable, and since 2002, only three or possibly four atoms of the isotope 294Uuo have been detected.
• therefore, next to no experiments can be one with it, but a lot of theories have come about.
• it may possibly not be a noble gas, unlike all the other Group 18 elements. It was formerly thought to be a gas but is now predicted to be a solid under normal conditions due to relativistic effects.
• Until the 1960s ununoctium was known as eka-emanation (emanation is the old name for radon)
• the element was to be called ununoctium,[35] a systematic element name, as a placeholder until the discovery of the element is confirmed and the IUPAC decides on a name. No name has yet been officially suggested for the element. IUPAC said the ultimate name for all new elements should end in “-ium” (not “-on”, even if it is a noble gas)
• The stability of nuclei decreases greatly with the increase in atomic number after plutonium, the heaviest primordial element, so that all isotopes with an atomic number above 101 decay radioactively with a half-life under a day, with an exception of dubnium-268.
• categories now only talk about “predicted compounds” so it is all guesswork.
|


|



![]()

![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()


![]()

![]()